Carbon and its Compounds
Revision Notes on Carbon and its Compounds
Two or more elements combine to form compound. There are two types of compounds- Organic Compound and Inorganic Compounds. Organic compounds are the one which are made up of carbon and hydrogen.Covalent Bond
The bond formed by sharing a pair of electrons between two atoms are known as Covalent Bond. Carbon forms covalent bond. Carbon exists in two forms- as free state and as combined state. Free form of carbon is found in graphite, diamond and fullerene. In combined state, carbon exists as Carbon-dioxide, Glucose, Sugar etc.Allotropes of Carbon
Different forms of an element that has same chemical properties but different physical properties are known as Allotropes. There are three allotropes of carbon- diamond, graphite and fullerene.Diamond
Diamond exits as three-dimensional network with strong carbon-carbon covalent bonds. Diamond is hard in nature with high melting point. It shines in presence of light and it is a bad conductor of electricity. The most common use of diamond is in making jewellery. It is also used in cutting and drilling tools.Graphite
Graphite is made from weak van der wall forces. Each carbon atom is bonded with other three carbon atoms in order to form hexagonal rings. It serves as good conductor of heat and electricity. It is used as dry lubricant for machine parts as well as it is used in lead pencils.Fullerene
It is a hollow cage which exits in the form of sphere. Its structure is similar to fullerene. But along with hexagonal rings, sometimes pentagonal or heptagonal rings are also present.Fig.1 Structure of fullereneTwo Important Properties of Carbon
Catenation and tetravalency are the two important properties of carbon. Catenation is a property of carbon by which carbon atoms can link one another via covalent bond and can form long chains, closed ring or branched chains etc. Carbon atoms can be linked by single, double or triple bonds. Carbon has a valency of 4 due to which it is known to have tetravalency. Due to this one carbon atom can bond with other 4 carbon atoms, with other atoms also such as Oxygen, Nitrogen etc.Hydrocarbons
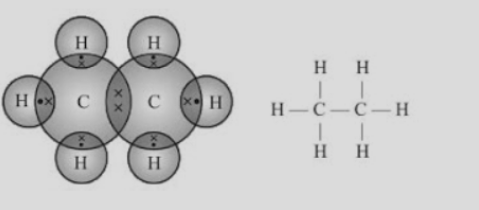
Compounds which are made up of carbon and hydrogen they are known as Hydrocarbons. There are two types of hydrocarbons found - Saturated Hydrocarbons and Unsaturated Hydrocarbons. Saturated Hydrocarbons consist of single bonds between the carbon atoms. For Example, Alkanes. Alkanes are saturated hydrocarbons represented by a formula, CnH2n+2.Unsaturated Hydrocarbons are the one with double or triple bonds between the carbon atoms. For Example, Alkenes and Alkynes. Alkenes are represented as CnH2n whereas alkynes are represented as CnH2n-2. Some saturated hydrocarbons and unsaturated hydrocarbons are represented as -Fig.2. Saturated hydrocarbonsFig. 3. Unsaturated hydrocarbonsStructure of hydrocarbons can be represented in the form of electron dot structure as well as open structures as shown below-Fig.4. Electron dot structure and open structure of ethaneFig.5. Electron dot structure and open structure of ethyneCarbons Compounds based on the basis of structure
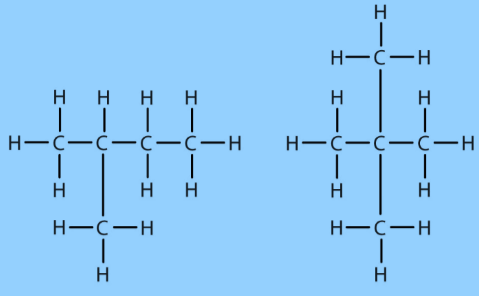
Carbon Compounds can be classified as straight chain compounds, branched chain compounds and cyclic compounds.They are represented as -Fig.6. Straight chain carbon compoundFig.7. Branched chain compoundsFig.8. Cyclic carbon compoundsFunctional Groups
One of the hydrogen atoms in hydrocarbon can be replaced by other atoms according to their valencies. The atoms which decides the properties of the carbon atoms, are known as Functional Groups. For Example, Cl, Br, -OH, Aldehyde, Ketone, Carboxylic Acid etc.Homologous Series
Series of compounds in which same functional group substitutes for the hydrogen atom in a chain of carbon.Fig.9. Homologous seriesNomenclature of Carbon Compounds
- First of all, identify the number of carbon atoms in compounds. And in it identify the longest chain
- Then functional group can be indicated by suffix or prefix.
- Cyclic hydrocarbon is designated by prefix cyclo.
- If there are two or more different substituents they are listed in alphabetical order
- If the same substituent occurs more than once, the location of each point on which the substituent occurs is given

Fig.10. Different functional groups
Chemical Properties of Carbon Compounds
Combustion
Carbon along with its compound is used as a fuel as it burns in presence of oxygen to release energy. Saturated hydrocarbons produce blue and non-sooty flame whereas unsaturated hydrocarbons produce yellow sooty flame.
CH4 + 2O2 → CO2 + 2H2O
Oxidation
Alcohol can be oxidized to aldehydes whereas aldehydes in turn can be oxidized to carboxylic acid. Oxidizing agent such as potassium permanganate can be used for oxidation.

Addition Reaction
Hydrogenation of vegetable oil is an example of addition reaction. Addition of hydrogen in presence of catalyst such as nickel or palladium. This converts oil into ghee.

Substitution Reaction
When one atom in hydrocarbon is replaced by chlorine, bromine, etc. this is known as Substitution Reaction.

Important Carbon Compounds: Ethanol and Ethanoic Acid
Ethanol is a volatile liquid with low melting point. It reacts with sodium to form sodium ethoxide.

This above reaction is used to test the presence of ethanol by the evolution of hydrogen gas.
Dehydration of ethanol in presence of hot sulphuric acid forms alkene.

Ethanoic acid is a colourless liquid. When pure ethanoic acid freeze like ice, it is known as Glacial Acetic Acid. It is formed at a temperature of about 16.6 degree centigrade
Ethanoic Acid/Acetic acid when reacts with ethanol it forms an ester. Ester can be identified by its sweet smell.

Reaction of ester with strong base is used to form soap. This is known as Saponification. Acetic acid also reacts with strong base to form sodium acetate and water.
NaOH + CH3COOH + CH3COONa + H2O
Soaps and Detergents
Sodium or potassium salt of carboxylic acid is known as Soap. They work most effectively in soap water. Detergents are sulphonate or ammonium salt of long chain of carboxylic acid. They can work effectively on soft as well as hard water.
Cleansing Action of Soaps and Detergents
Cleansing action of soaps and detergents is due to ability to minimize the surface tension of water, to emulsify oil or grease and to hold them in a suspension of water. When soap dissolves in water, it forms soap anions and soap cations. The hydrophobic part of soaps and detergents are soluble in grease and hydrophilic part is soluble in water.
Soap and Micelle Formation
When dirt and grease are mixed with soap water, soap molecules arrange them in tiny clusters known as Micelle. The hydrophilic part sticks to the water and form outer surface of the micelle and hydrophobic part binds to oil and grease.




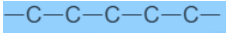

This is to inform that hydrochloroquune is effected in the case of Corona all that revealed information is effected only malaria case .and that medicine is given to the patient is not right
ReplyDeletehttps://www.allayurvedic.org/2020/02/त्वचा-के-सभी-चर्म-रोगों-का.html
ReplyDeletehttps://www.chemistrywave.com/2020/05/class-xi-study-material-physics.html?m=1
ReplyDeleteChemistry k notes
ReplyDeleteBiology k notes
ReplyDeletePhysics k notes
ReplyDelete